The hydrogen solubility in 42 organic compounds including alcohols, aldehydes, carboxylic acid, esters and ethers, glycols, n-alkanes, and water is investigated. For this purpose, the Henry’s. Expand. By using the equations found in the paper you have sent, I could obtain the following solubility of hydrogen ( ppm) in methanol, ethanol, and n-hexane: Methanol: 1st method: 8.030401. 2nd method.
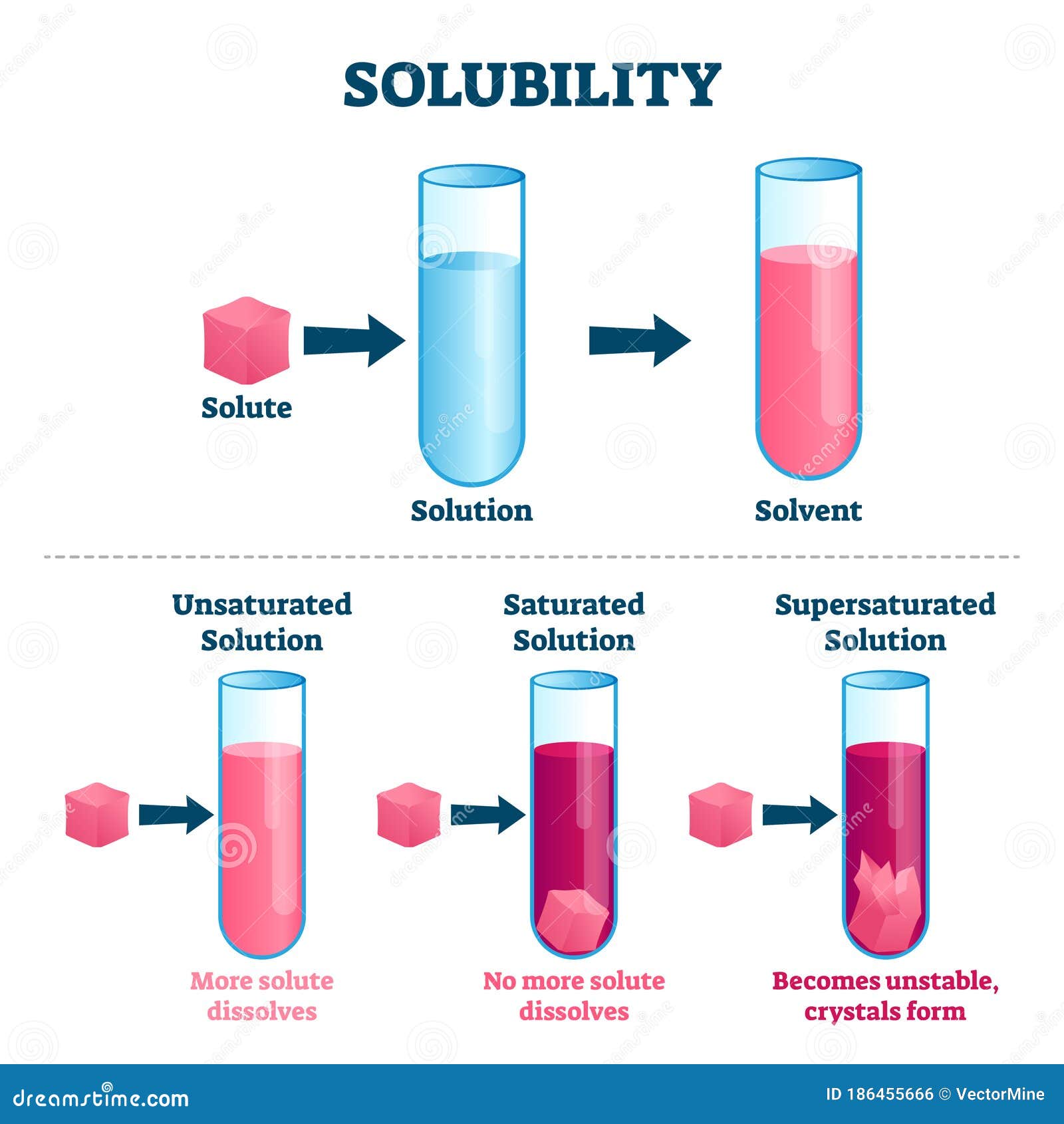
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Scheme Stock Vector

Shows the list of common organic solvents, their formula and melting… Download Scientific
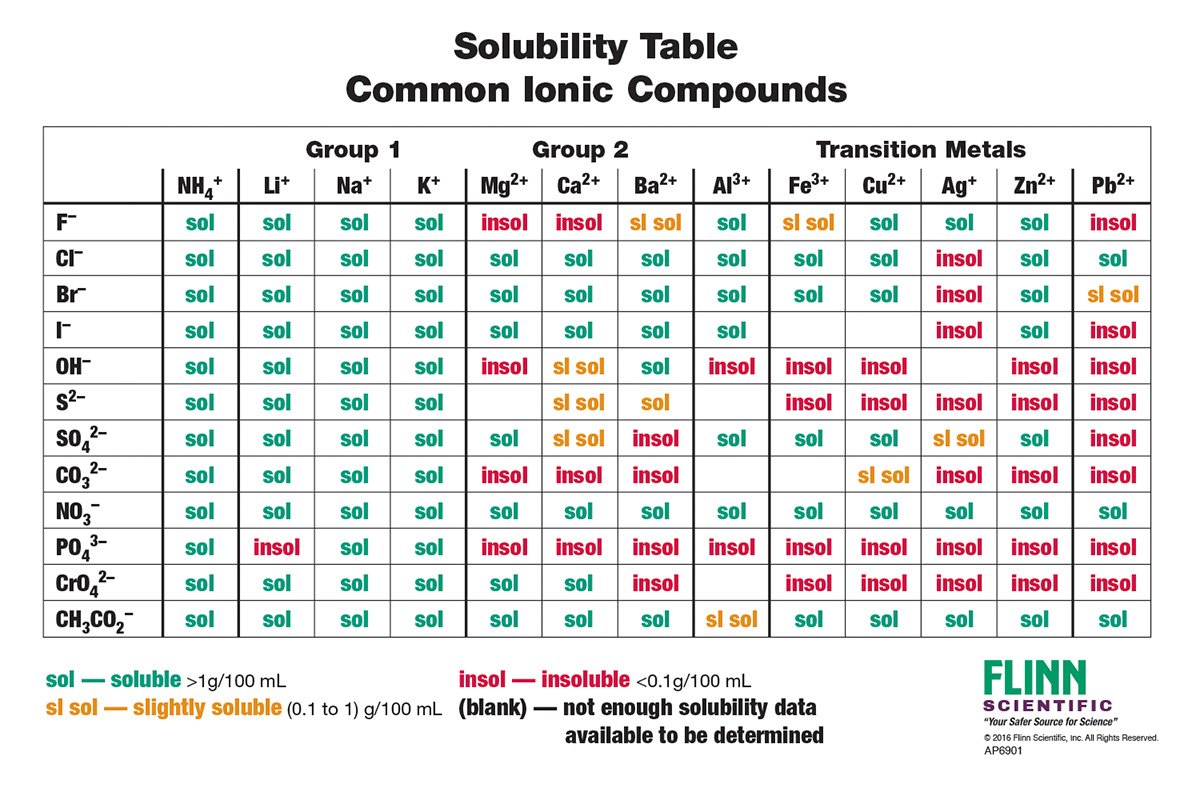
Solubility Rules Charts for Chemistry

Solubility In Water Chart
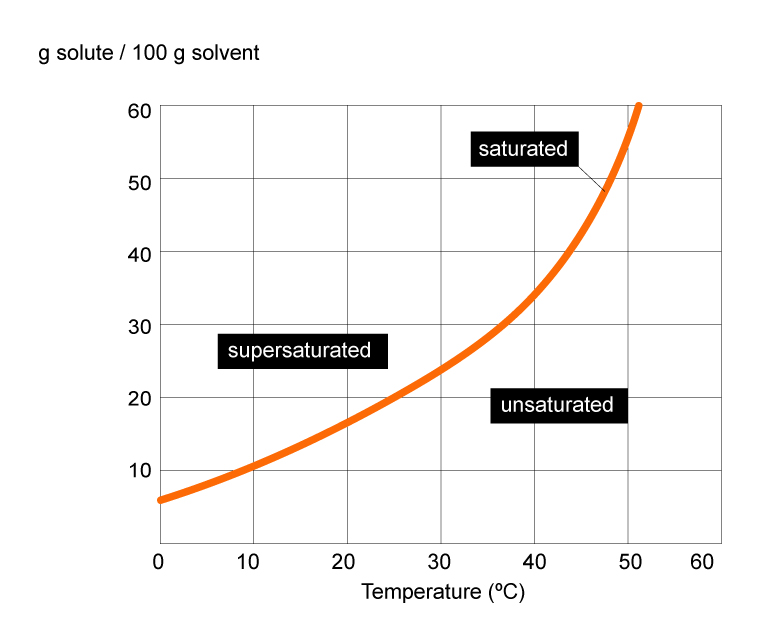
Solubility Surfguppy Chemistry made easy for visual learners
![Hydrogen solubility in pure magnesium [21].](https://www.researchgate.net/publication/272075805/figure/download/fig8/AS:978969029529601@1610415813054/Hydrogen-solubility-in-pure-magnesium-21.png)
Hydrogen solubility in pure magnesium [21]. Download Scientific Diagram

Solubility of Organic Compounds Chemistry Steps
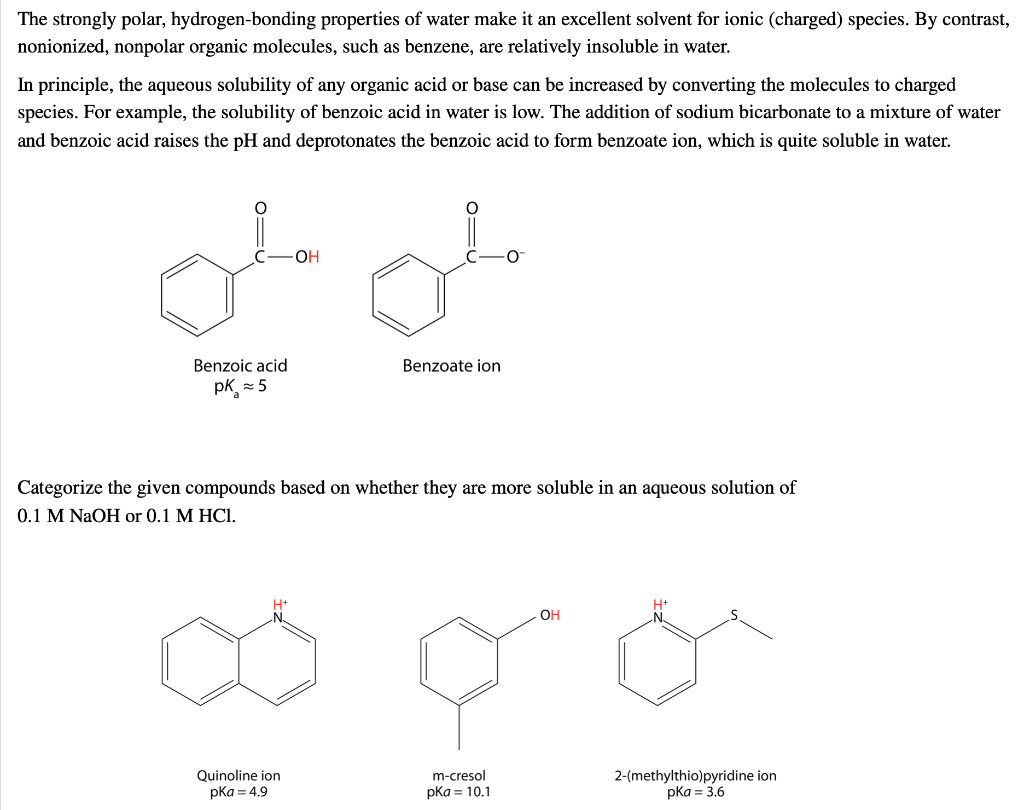
Solved The strongly polar, hydrogenbonding properties of
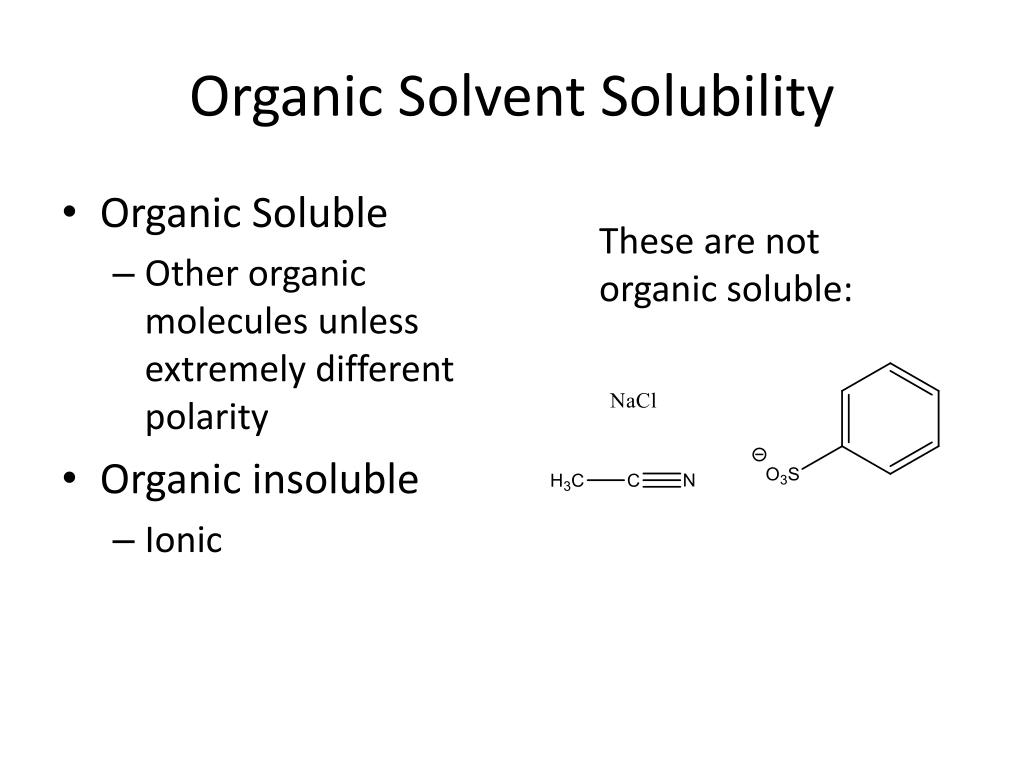
PPT Solubility and Extraction PowerPoint Presentation, free download ID2528518

Butanol and water partition and solubility data in various organic… Download Scientific Diagram

Solubility Rules Pathways to Chemistry

Solubility of Electrolytes in Organic Solvents SolventSpecific Effects and IonSpecific

Summary of Hydrogen Solubility for Adsorbates at 303.15 K Download Scientific Diagram
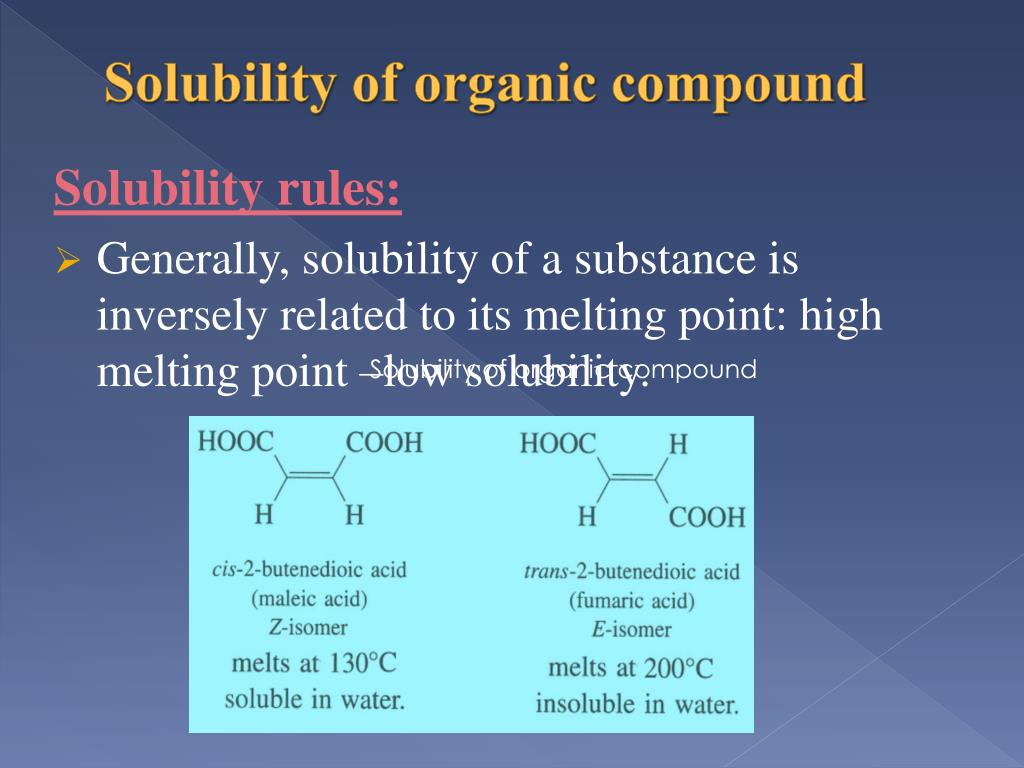
PPT Solubility of organic compound organic chemistry II lab PowerPoint Presentation ID3105359
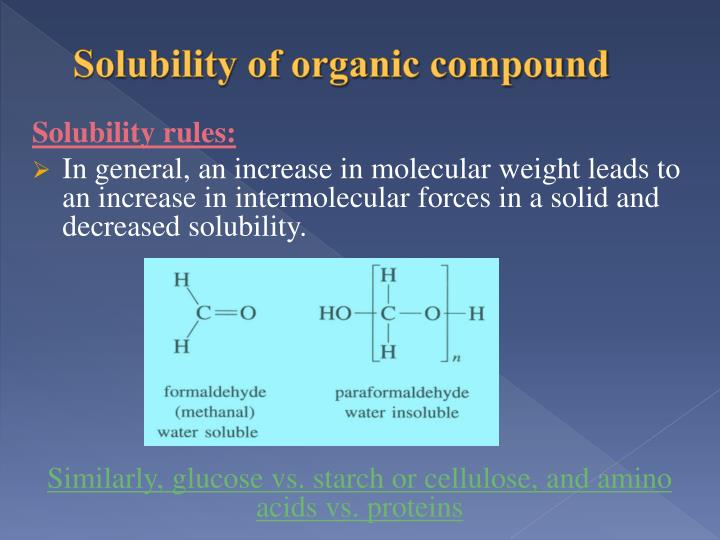
PPT Solubility of organic compound organic chemistry II lab PowerPoint Presentation ID3105359
PPT Solubility PowerPoint Presentation, free download ID6260427

Solubility of Organic Compounds Chemistry Steps

13.3 Factors Affecting Solubility Chemistry LibreTexts

Hydrogen Solubility in Hydrocarbon and Oxygenated Organic Compounds Journal of Chemical
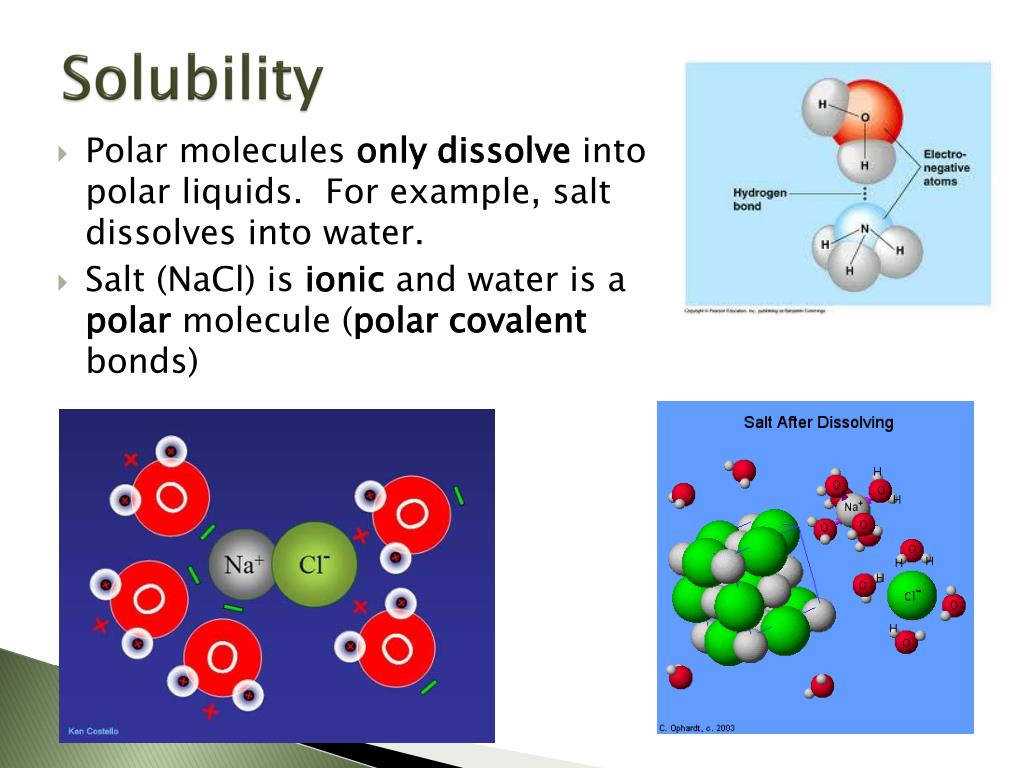
PPT Solubility PowerPoint Presentation, free download ID2493612
The solvatochromicity of established solvatochromic UV/Vis probes, which appear to be sensitive to the so-called hydrogen bond donor (HBD) property of the solvent, is analysed using the hydroxyl group density of alcoholic solvents D HBD as a physical parameter in comparison to the pKa, the chemical benchmark for acidity. Reichardt’s dye B30, Kosowers Z-indicator 1-ethyl-4-(methoxycarbonyl.. solvents can remarkably a˜ect the hydrogen solubility as a thermodynamic quantity. Increasing pressure and temperature have an increasing impact on the solubility of gases.

